A Point-Of-Care Urine Osmometer for Nocturia Evaluation
KEY INFORMATION
Healthcare - Medical Devices
TECHNOLOGY OVERVIEW
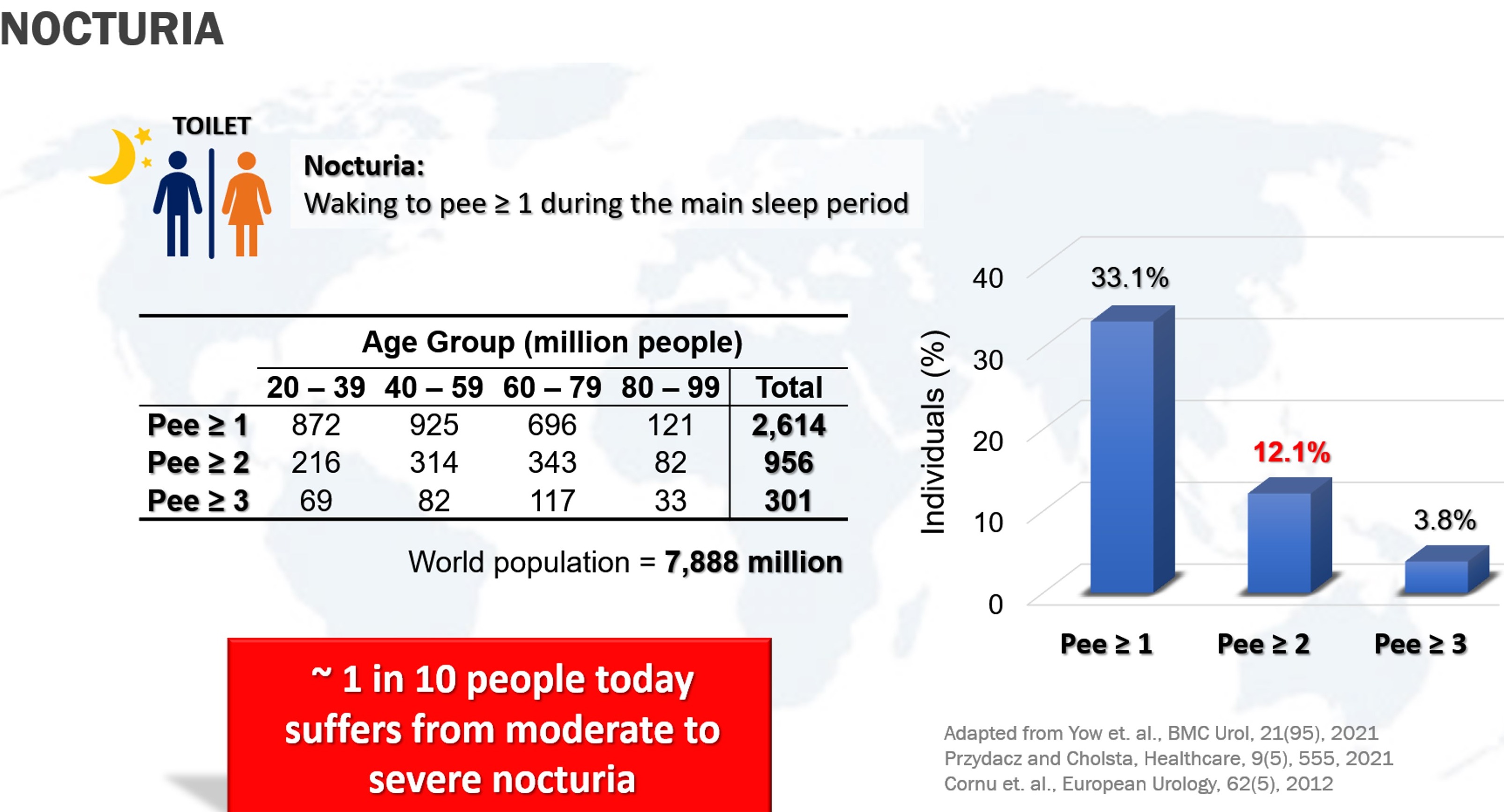
Nocturia is a common urinary complaint where one experience interruption of sleep with one or more times at night to void. The current assessment of nocturia is primarily clinical and relies mainly on patients documenting their own bladder diary to complete a Frequency-Volume Chart (FVC) of volume and times of urination between a 24-to-72-hour period. This conventional way of assessment is a manual and inadequate way of diagnosing nocturia. An individual is diagnosed with nocturnal polyuria if the total urine output at night exceeds one-third of total daily output.
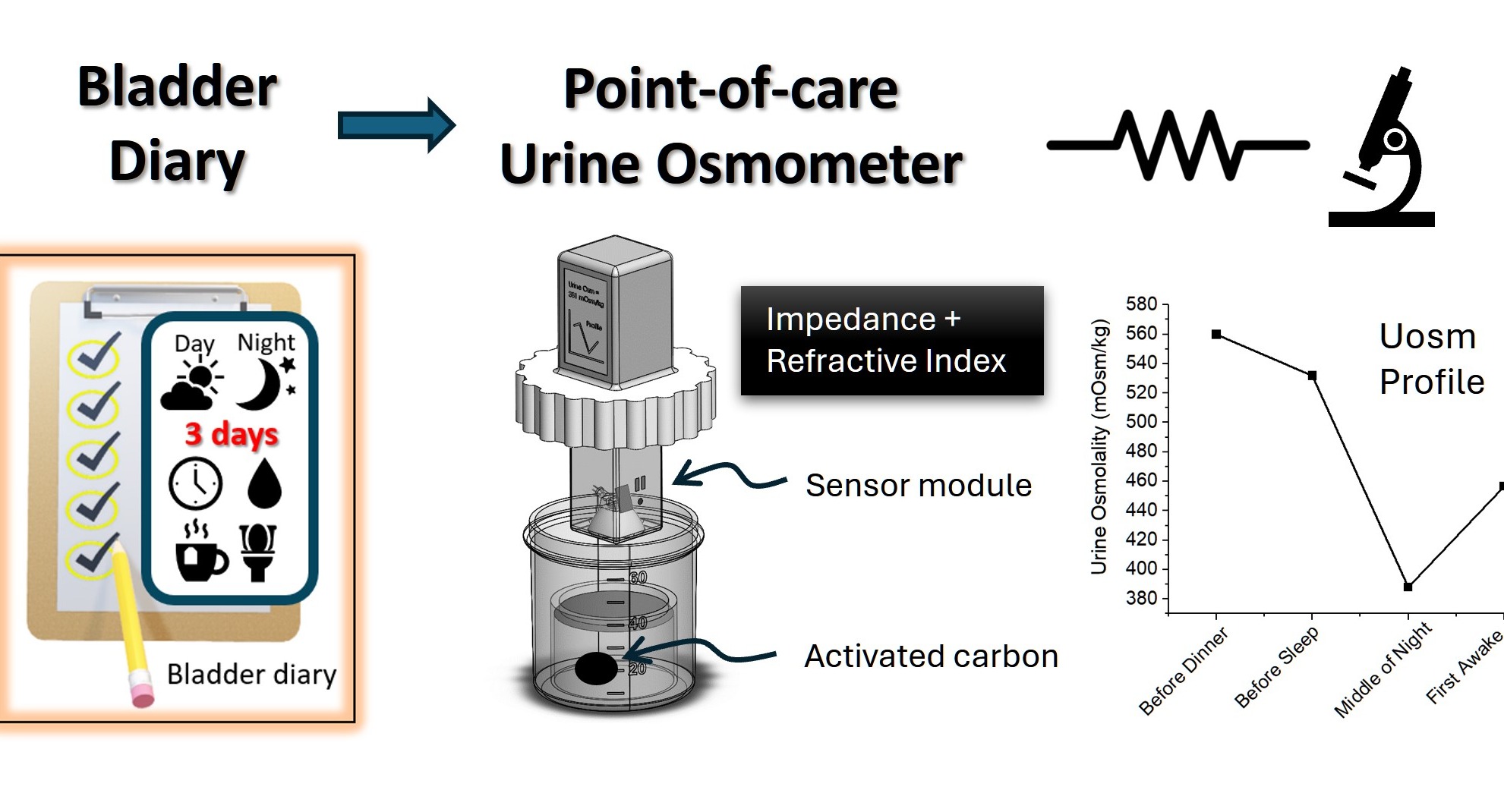
This technology has developed a point-of-care urine osmometer for the monitoring and profiling of day- and night-time variation in urine osmolality, providing clinicians with valuable insights to facilitate more accurate, objective diagnosis and personalized treatment plans for nocturia. This device is portable and attached with a readout sensor, designed to be user friendly.
The technology owner is seeking collaborations with:
Medical Institutions and Healthcare Providers: To facilitate clinical validation, implementation, and integration of the portable urine osmometer into routine diagnostic practices for nocturia and other related conditions.
Device Manufacturers: To miniaturize the prototype, scale up production, ensure quality control, and help bring the device to market.
Research Institutes: To collaborate on further R&D , particularly in optimizing the technology and exploring additional applications, such as hydration monitoring for athletes and military personnel.
Data Analytics Partners: To develop advanced software and algorithms for precise data interpretation, which can enhance the device’s diagnostic capabilities and provide more personalized treatment options.
TECHNOLOGY FEATURES & SPECIFICATIONS
Current state-of-the-art for urine osmolality measurement uses a freezing point osmometer, which is often bulky, expensive, and requires specialized training to operate. To address these limitations, a portable urine osmometer that uses impedance measurement coupled with refractive index measurement and activated carbon absorption was developed. This innovative approach provides a quick and accurate measurement of urine osmolality. In a clinical trial involving 225 urine samples, an accuracy of 94.4 ± 5.0% was achieved, comparable to the gold standard freezing point osmometer. The device's compact design allows for easy home use, making it ideal for patients with nocturia to monitor their condition without frequent clinic visits.
POTENTIAL APPLICATIONS
The current pain point in diagnosing and managing nocturia lies in the absence of an objective and quantitative method. Current clinical diagnosis relies heavily on a cumbersome 3-day bladder diary, which is insufficient but also results in inaccuracies and poor patient compliance. Consequently, urologists are left with the challenging task of providing a diagnosis for the underlying cause of nocturia which may lead to a trial-and-error approach on the medications prescribed. This results in suboptimal management of the condition, leading to unresolved patient discomfort and dissatisfaction. The proposed solution aims to revolutionize the approach to diagnosing and treating nocturia by introducing a portable point-of-care device for urine osmolality profiling. The portable urine osmometer is not only useful for diagnosing nocturia but has potential applications in assessing, monitoring hydration status and renal function in various populations, particularly in athletes, elderly, and military personnel.
Market Trends & Opportunities
The market potential for portable urine osmometer, particularly in addressing nocturia, is significant. The global market for nocturia-related drugs is projected to grow at a compound annual growth rate (CAGR) of 6.1%, highlighting the increasing demand for effective nocturia management solutions. Given the high prevalence of nocturia, with approximately 1 in 10 people experiencing moderate to severe symptoms, and the current limitations of diagnostic methods, there are substantial opportunities for a portable urine osmometer. This device offers a cost-effective and accessible alternative to the traditional freezing point osmometer.
Unique Value Proposition
This technology offers a more precise measurement in assisting physicans on the diagnosis for nocturnal polyuria and providing significant improvement to patient protocol. Besides that, the technology enhances several UVP over the conventional methodology which includes:
- Providing a comprehensive day and night urine osmolality profile in a home setting with an objective and quantitative readout over the current reliance on subjective bladder diaries.
- Compared to existing devices in the market, this device has a much smaller footprint than a laboratory based freezing point osmometer.
- The device is simple to use and enables a cheaper manufacturing cost.
- It has a short turnaround time with a 3-minutes readout duration.
- An affordable consumer device as the consumable per test is a plastic container pre-filled with activated carbon and a disposable urine container.